WHY THIS STUDY?
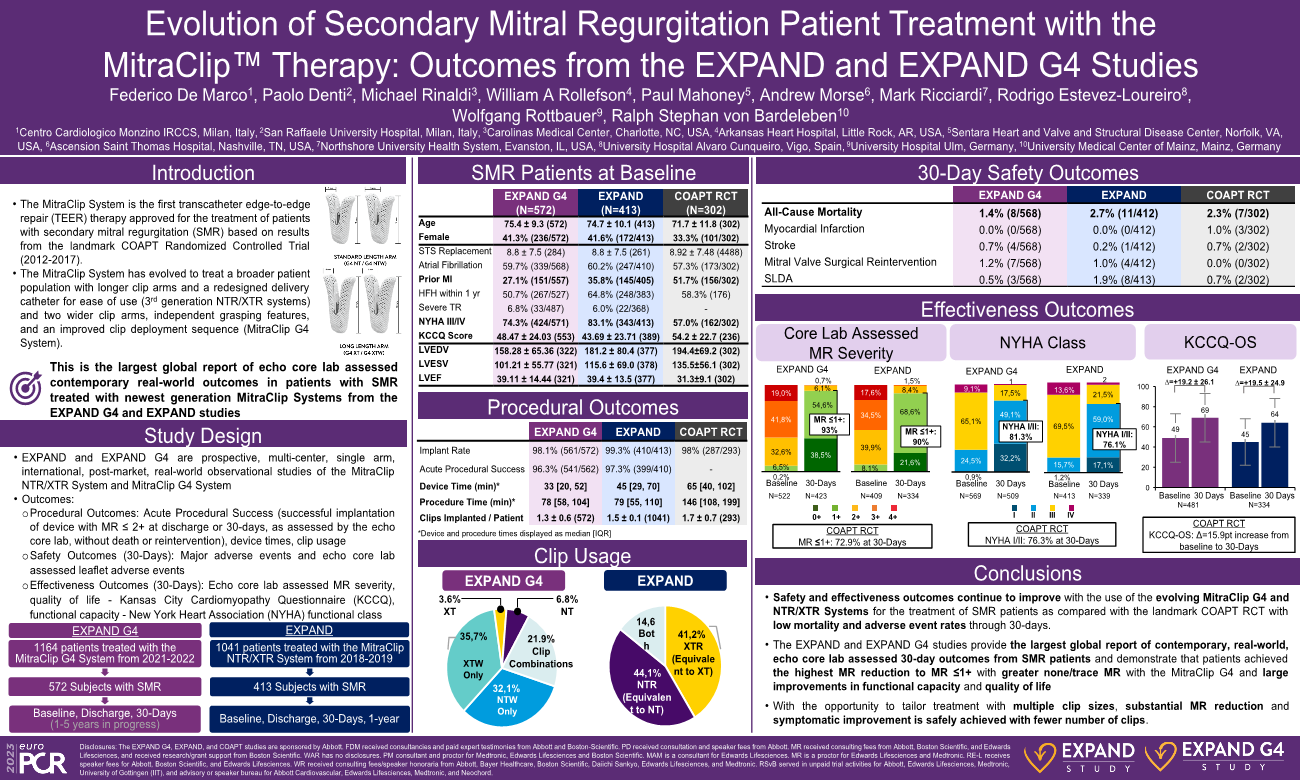
The MitraClip? System is the first transcatheter edge-to-edge repair(TEER) therapy approved for the treatment of patients with heart failure and secondary mitral regurgitation(SMR) on guideline-directed medical therapy. Approval(FDA 2019) and IIa recommendations by the ACC/AHA and ESC guidelines were based on results from the landmark COAPT RCT that enrolled patients from 2012-17. Since the 1st generation MitraClip devices used in the COAPT RCT, the MitraClip System has evolved to treat broader patient populations with longer clip arms and a redesigned delivery catheter for ease of use in the 3rd generation NTR/XTR systems and two wider clip arms, independent grasping features, and an improved clip deployment sequence in the MitraClip G4 System. This is the largest global report of contemporary, real-world outcomes in patients with SMR treated with the newest generation MitraClip Systems from the EXPAND G4 and EXPAND studies.
HOW WAS IT EXECUTED?
EXPAND G4 and EXPAND are prospective, real-world, global, multi-center, single-arm studies that have enrolled 1164(2021-22) and 1041(2018-19) subjects treated with the MitraClip G4 and NTR/XTR Systems, respectively, in Europe, Canada, the U.S, Middle East, and Japan. Outcomes include echocardiography measurements assessed by independent echocardiography core labs, procedural, and 30-day MR reduction, New York Heart Association(NYHA) Functional Class, Kansas City Cardiomyopathy Questionnaire(KCCQ) score, changes in heart failure medications, and leaflet and major adverse events. Baseline characteristics and 30-day outcomes are reported for subjects with SMR in EXPAND G4 and EXPAND.
WHAT WERE THE ESSENTIAL RESULTS?
572 subjects in EXPAND G4 and 413 subjects in EXPAND had SMR. Compared to subjects in COAPT, subjects in EXPAND G4 and EXPAND were slightly older(75±9y EXPAND G4, 75±10y EXPAND vs 72±12y COAPT) and more often female(41%, 42% vs 33%) with smaller baseline left ventricular end-diastolic(158±65, 181±80ml vs 194±69ml) and end-systolic volumes(101±56, 116±69ml vs 136±56ml). At baseline, subjects in EXPAND G4 and EXPAND had higher proportions with NYHA Class III/IV(74%, 83% vs 57%) and lower KCCQ scores(48±24, 44±24pts vs 54±23pts) compared with subjects in COAPT. Subjects with SMR were treated with fewer clips(1.3±0.6, 1.5±0.1 vs 1.7±0.7) and had high implant(98%, 99%) and acute procedural success rates(96%, 97%) with lower device times(41±34, 55±40min vs 83±81min) compared to COAPT. At 30 days, MR reduction to mild or less was achieved in 93% and 90% of subjects in EXPAND G4 and EXPAND, respectively, compared with 69% in COAPT. From baseline to 30 days, improvements in functional capacity were achieved with NYHA Class I/II in 81% and 76% of subjects and a +19±26 and +19±25pt increase in KCCQ score with low all-cause mortality rates of 1.4% and 2.7% in EXPAND G4 and EXPAND, respectively. Additional outcomes including heart failure medications, leaflet adverse events, and major adverse events will be reported in this presentation.
WHY IT IS IMPORTANT?
With the evolution of TEER for the treatment of patients with SMR, safer and more effective outcomes are achieved with the use of the MitraClip G4 and NTR/XTR Systems as compared with prior RCTs. To date, this is the largest global report of 30-day outcomes from subjects with SMR and shows the highest MR reduction using core lab adjudication with large improvements in functional capacity and quality of life despite an older and sicker patient population in a contemporary and real-world setting.
PLEASE LIST THE DEVICE(S)/TECHNOLOGY(IES) INVOLVED IN THIS TRIAL
Abbott MitraClip? System