AIMS:
Urgent coronary artery bypass grafting (CABG) within two days of ticagrelor administration is associated with high rates of major bleeding (>25%) and high rates of perioperative mortality in those with major bleeding (>9%). Intraoperative extracorporeal haemoadsorption is an approved therapy to potentially reduce perioperative bleeding by active ticagrelor removal. The International Safe and Timely Antithrombotic Removal (STAR) registry is collecting data on real-world use and outcomes in this application (ClinicalTrials.gov identifier: NCT05077124).
METHODS AND RESULTS:
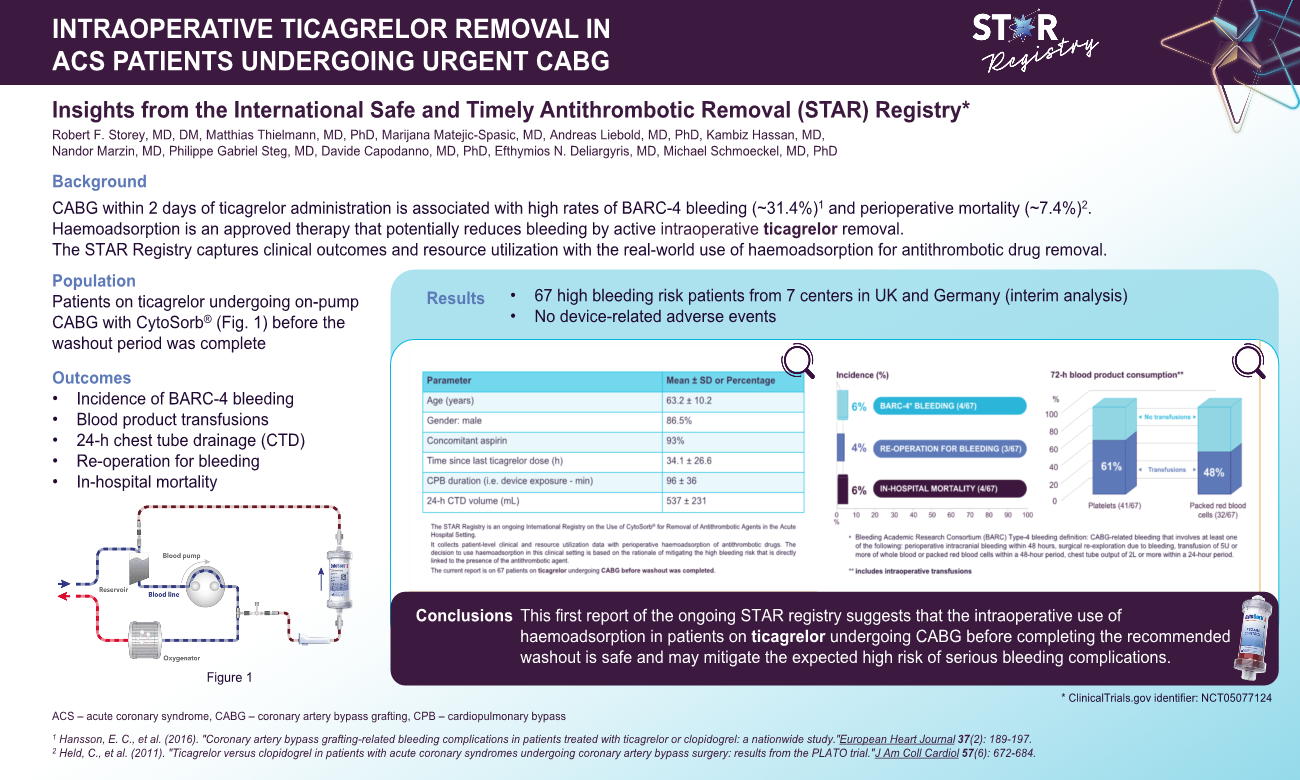
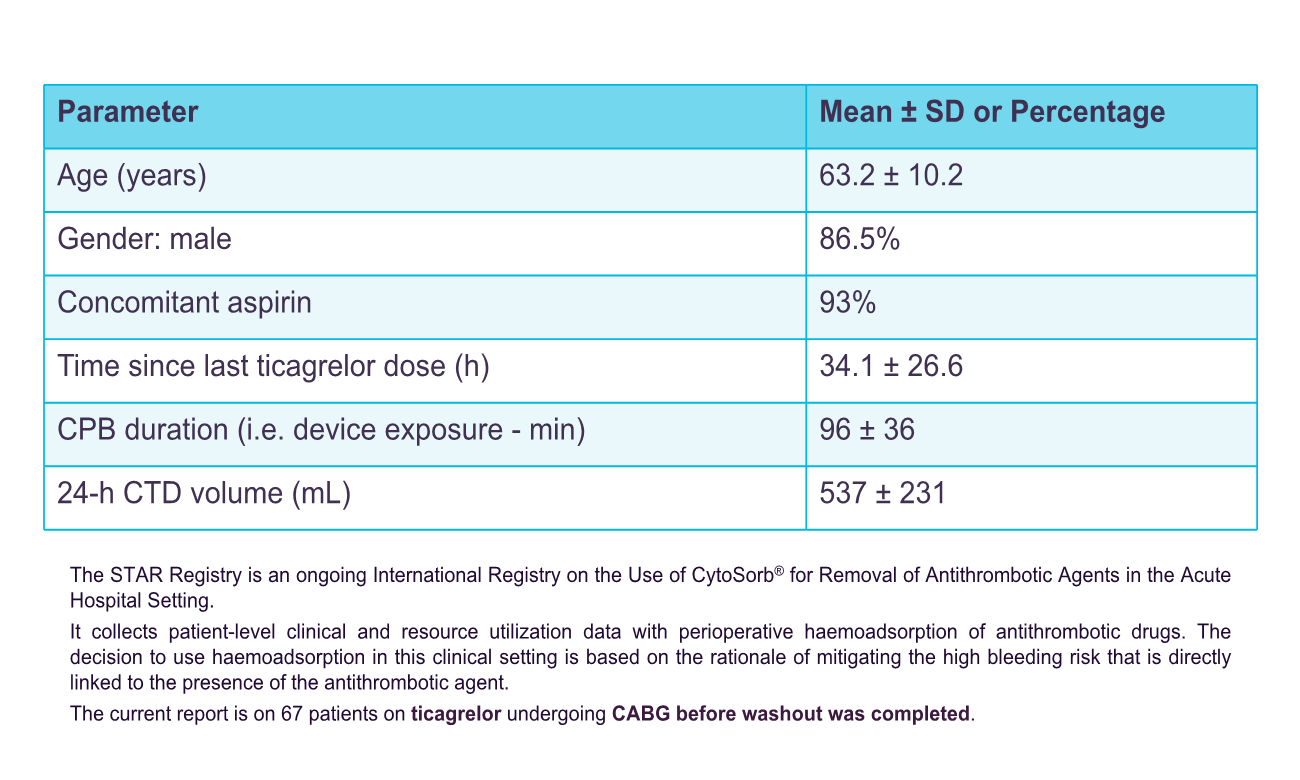
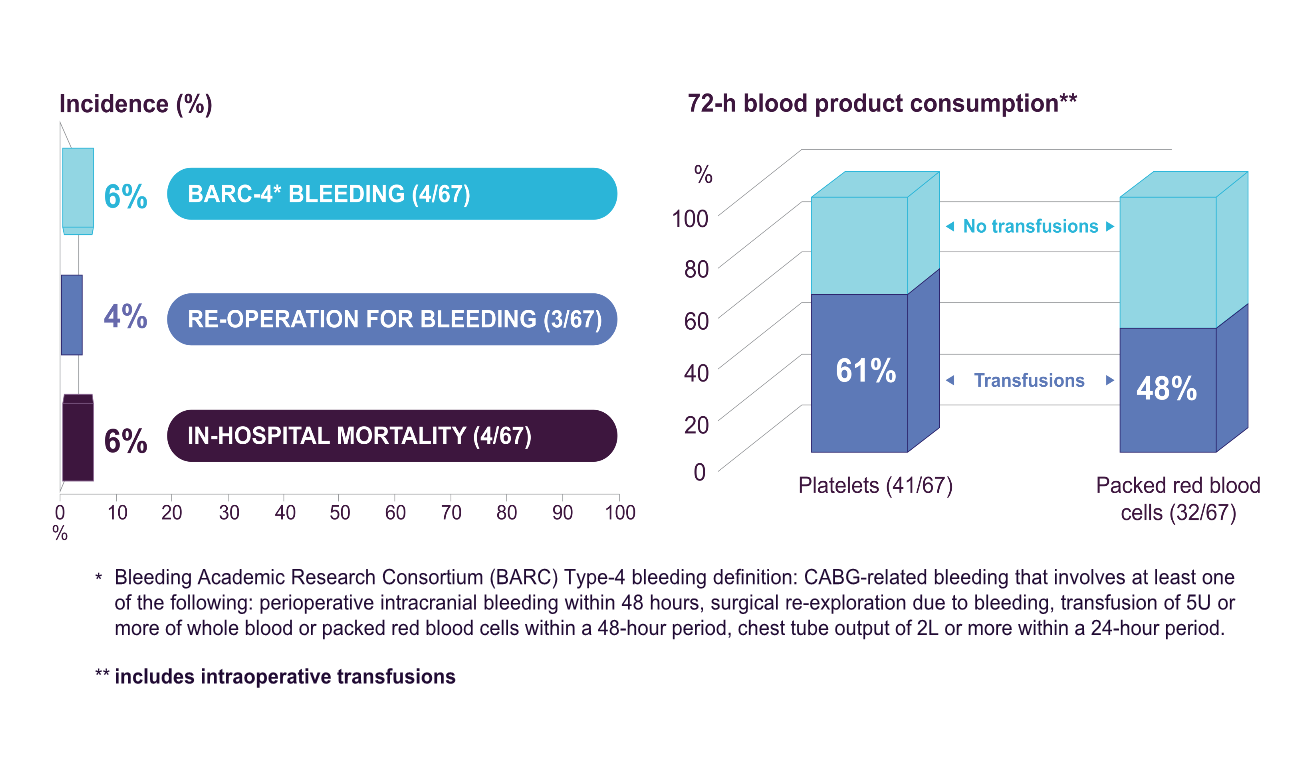
Retrospective and prospective data were collected on patients on ticagrelor undergoing urgent CABG plus intraoperative haemoadsorption. Perioperative bleeding was assessed according to the Bleeding Academic Research Consortium (BARC) definition, need for perioperative blood product transfusions, and 24-hour chest-tube drainage (CTD). The current analysis comprises of 67 patients from 7 centres in the UK and Germany. Mean age was 63.2±10.2 years, 86.5% were male and 93% were also on aspirin. All patients underwent isolated on-pump CABG at a mean 34.1±26.6 hours since last ticagrelor dose. The haemoadsorption device was integrated in the cardiopulmonary bypass circuit and was active for a mean duration of 96±36 min. In-hospital mortality was 6% (4/67), rate of BARC-4 bleeding was 6% (4/67) and need for re-operation for bleeding was 4% (3/67). Platelet transfusions were administered to 61% of patients (41/67), while only 1 patient required >5 units of packed red blood cells. Mean 24-hour CTD volume was 537±231mL, with no patient exceeding 2,000mL. Device use was assessed as easy and safe by all participating centres without any device-related adverse events reported.
CONCLUSION:
This first report of the ongoing STAR registry suggests that the use of intraoperative haemoadsorption in patients on ticagrelor undergoing urgent CABG is safe and may mitigate the expected high risk of serious bleeding complications.